Heat-treated probiotic FK-23
FK-23, a heat-treated probiotic material, is useful for maintaining true daily health. FK-23 was born through the research and development, and a number of patents were granted to Nichinichi pharmaceutical Co., Ltd. We established a technique for heat-killing method by which the immunity is enhanced up to approximately three-fold compared to the live bacteria.

FK-23 material (Heat-treated probiotic FK-23 powder)
1. Trait
FK-23 material is powder of heat-killed Enterococcus faecalis FK-23 that is derived from pure cultivation. This food material contains over 4000 billion of the bacterial cells per 1 gram. Any extending agents and additives are not included in this material.
2. Quality Standards
| Standard item | Standard value | Test method |
|---|---|---|
| Dry weight percentage | ≦ 5.0% | Atomospheric heat dry |
| Heavy metal (Pb) | ≦ 20ppm | Colorimetric method with sodium sulfate |
| General bacteria count | ≦ 3000 cells/g | Standard agar plate culture method |
| Coliform | Negative | BGLB method |
| Fungi count | ≦ 300 cells/g | Petrifilm method |
3. Examples of Food Labels
Probiotic, Heat-treated probiotic powder, Heat-treated probiotic, etc
Product Trait
1. About Enterococcus faecalis
Enterococcus faecalis is a regular component of the intestinal flora in healthy young adults. It is known that Enterococcus faecalis is deeply involved in maintaining human health.
2. About FK-23
Enterococcus faecalis FK-23 (hereafter abbreviated to FK-23) was screened at Nichinichi pharmaceutical Co., Ltd. Heat-treating of FK-23 enhances the immune-activating effect up to three-fold compared to the live bacteria.
3. Function of FK-23
FK-23 shows an excellent absorptive property in human body, and works on immune cells in the body. Therefore, various values are expected.
| Patent | [1] Immune-activating effect | Improve the function of leukocytes and increase the number of leukocytes |
|---|---|---|
| Patent | [2] Anti-tumor effect | Reduce the size of tumor |
| Patent | [3] Suppression of side effects of anticancer drugs | Suppress the growth of tumor and reduce the side effects of anticancer drugs |
| Patent | [4] Improvement of hepatitis C | Improve the liver functions |
| Patent | [5] Anti-pathogenic infection effect | Prevent the pathogenic infectious diseases |
4. Feature of FK-23
- Contain over 4000 billion of the bacterial cells per 1 gram
- Original treating method (Granted patent)
- Stable physicochemically
- Stable preservation (Quality guarantee for two years)
- Light colored powder
- 100% Enterococcus faecalis (No extending agents and additives)
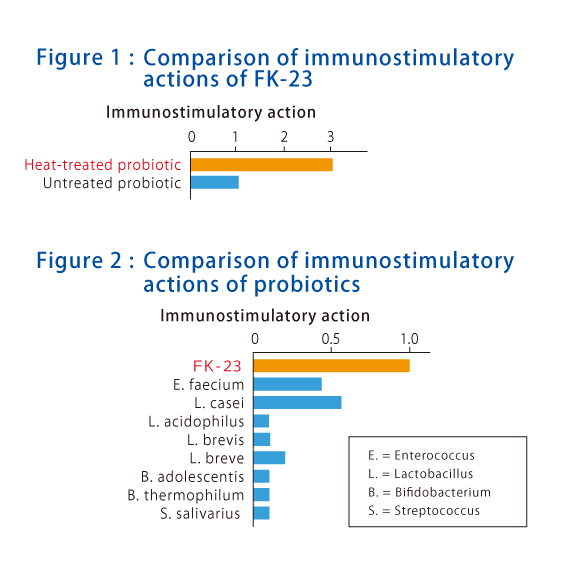
Figure 2 shows the comparison of immune-activating effect of various types of heat-treated probiotic. It has been cleared that FK-23 (orange) shows higher immune-activating effect than other lactic acid bacteria (blue: Enterococcus faecium, Lactobacillus casei, Lactobacillus acidophilus, Lactobacillus brevis, Bifidobacterium breve, Bifidobacterium adolescentis, Bifidobacterium thermophilum, and Streptococcus salivarius).
5. Safety of FK-23
- No abnormality was observed in rec assay using Bacillus subtilis and mutagenicity test (micronucleus assay) in mice.
- In the subacute toxicity test using rats at high dose of FK-23 (3000?5500 mg/kg), no abnormality was observed on general conditions, body weight, urine analysis, blood analysis, etc. (for human, usual intake is about 20-50 mg/kg).
- In clinical test in human adults (intake of 8.1 g FK-23 per day for 4 weeks), no abnormality was observed on all general analyses such as blood pressure, biochemical test of blood, urine analysis, questionnaire and so on.
- Enterococcus faecalis FK-23 is confirmed to be safe probiotic bacteria because of the absence of multidrug resistance genes (VRE; vanC-1, vanC-2, and vanC-3).
6. Application of FK-23 to health foods and the references
Research on FK-23 was published to academic journals, presented to academic societies, and granted patents. FK-23 is able to be utilized for various purposes as health food materials not only for human but also for pets, as described below.
| Cancer |
|
|---|---|
| Immune activation (Aquaculture and stockbreeding) |
|
| Infectious disease Influenza Hepatitis C |
|
| Calming intestinal disorders |
|



